What is the catalyst of a catalytic converter?
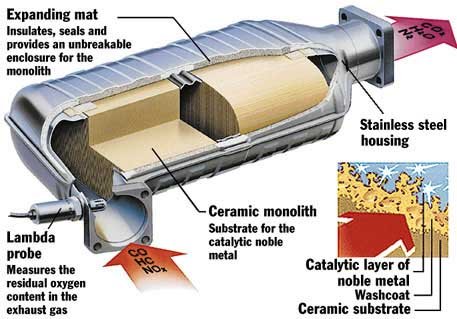
It is a thin coating of precious metals (platinum, palladium and rhodium) applied to the surface of the substrate – the inner lining of the converter; its function is to assist in the chemical reactions that are involved in reducing the emission levels.
Platinum (Pt), palladium (Pd), and rhodium (Rh) coat a ceramic honeycomb (or ceramic beads) contained within a metal casing that is attached to the exhaust pipe.
The catalytic converter’s honeycomb structure provides the maximum surface area on which reactions can take place while using the least amount of catalyst.
Related Questions
- What is a catalytic converter?
- What is the catalyst of a catalytic converter?
- What is the difference between “oxidation” and “three-ways’ converters?
- Can a bad catalytic converter cause a car to overheat? what causes a converter to become red hot?
- What causes a catalytic converter to become clogged?
Still need help ?
Contact our 24/7 support.

























